Prepare to conquer the CHEM 1211 ACS Final Exam with our comprehensive guide. This exam-focused resource unravels key concepts, problem-solving techniques, and study strategies to empower you for success.
Delve into the core principles and theories tested on the exam, along with practical applications that bridge theory and real-world chemistry. Unlock effective problem-solving strategies and avoid common pitfalls to tackle complex questions confidently.
Concepts and Theories
The ACS final exam for CHEM 1211 covers a wide range of fundamental concepts and theories in chemistry. These concepts form the foundation of chemical knowledge and are essential for understanding the behavior of matter at the molecular level.
The exam tests students’ understanding of:
- Atomic structure and bonding
- Chemical reactions and stoichiometry
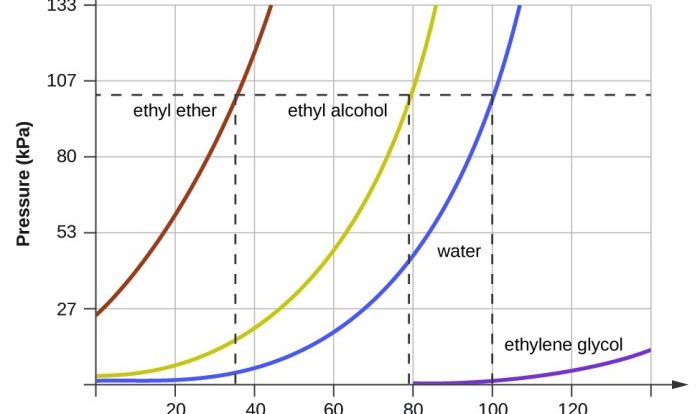
- States of matter and solutions
- Thermochemistry
- Equilibrium
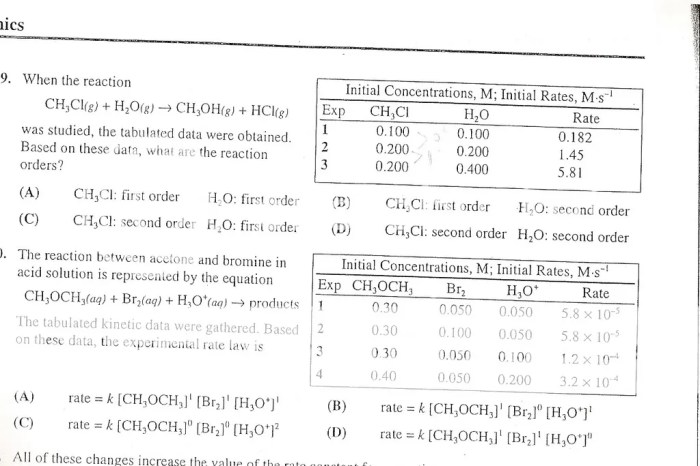
li>Kinetics
Atomic Structure and Bonding
The exam assesses students’ knowledge of the structure of atoms, including the arrangement of electrons in orbitals and the formation of chemical bonds. Key theories tested include:
- Quantum mechanics
- Valence bond theory
- Molecular orbital theory
Examples of how these concepts are applied in real-world chemistry include:
- Predicting the properties of new materials
- Designing drugs that target specific molecules
- Understanding the mechanisms of chemical reactions
Problem-Solving Strategies
Conquering ACS final exam questions requires effective problem-solving strategies. By adopting a systematic approach, you can break down complex chemistry problems and arrive at accurate solutions. Here’s a step-by-step guide to help you tackle these challenges:
Read and Analyze the Question
Begin by thoroughly reading the question. Identify the key concepts and what the question is asking. Note any given information and the desired output. This initial step ensures you have a clear understanding of the problem.
While studying for my Chem 1211 ACS final exam, I stumbled upon a curious word: “phonetics.” This led me down a rabbit hole of exploring words with phon in them , discovering a plethora of fascinating terms like “phonograph” and “phonemic.”
As I delve deeper into the intricacies of chemical reactions, I can’t help but appreciate the subtle connections between science and language, making my Chem 1211 ACS final exam preparation a surprisingly enriching experience.
Plan Your Approach
Based on your analysis, devise a plan to solve the problem. Consider the relevant concepts, equations, and formulas that apply. Artikel the steps you need to take to reach the solution.
Execute Your Plan
Carry out your plan systematically. Show all your work, including calculations, equations, and reasoning. Clearly indicate any assumptions or approximations you make.
Check Your Answer
Once you have a solution, check your answer. Ensure it makes sense and is consistent with the given information. If possible, verify your answer using a different approach or by consulting reliable sources.
Avoid Common Pitfalls
Be aware of common pitfalls and misconceptions. Avoid making assumptions that are not stated in the question. Pay attention to units and significant figures. Carefully interpret data and avoid overgeneralizing.
Experimental Techniques
Experimental techniques are crucial in chemistry as they enable scientists to investigate chemical phenomena, test hypotheses, and develop theories. The ACS final exam commonly assesses students’ understanding of various experimental techniques, including their principles, applications, and how to design and conduct experiments using these techniques.
Spectroscopy
Spectroscopy is a powerful tool for identifying and characterizing compounds by analyzing the interaction of electromagnetic radiation with matter. Various spectroscopic techniques, such as UV-Vis, IR, and NMR spectroscopy, are used to determine molecular structure, functional groups, and other chemical properties.
- UV-Vis Spectroscopy:Measures the absorption of ultraviolet and visible light by a sample, providing information about electronic transitions and chromophores present.
- IR Spectroscopy:Analyzes the absorption of infrared radiation, revealing the presence of specific functional groups and providing insights into molecular structure.
- NMR Spectroscopy:Utilizes the magnetic properties of atomic nuclei to determine the structure and dynamics of molecules, especially organic compounds.
Electrochemistry
Electrochemistry involves the study of chemical reactions that occur at the interface between an electrode and an electrolyte. Electrochemical techniques, such as cyclic voltammetry and potentiometry, are used to investigate redox reactions, determine electrode potentials, and study the behavior of electrochemical systems.
- Cyclic Voltammetry:Applies a varying potential to an electrode and measures the resulting current, providing information about redox processes and electrode kinetics.
- Potentiometry:Measures the potential difference between two electrodes to determine the concentration of ions in a solution or the equilibrium constant of a reaction.
Chromatography
Chromatography is a separation technique used to separate and identify components of a mixture based on their different physical and chemical properties. Various chromatographic techniques, such as HPLC and GC, are employed to analyze complex samples and quantify their components.
- HPLC (High-Performance Liquid Chromatography):Separates compounds based on their polarity and size, using a liquid mobile phase and a stationary phase packed in a column.
- GC (Gas Chromatography):Separates volatile compounds based on their boiling points and interactions with a stationary phase, using a carrier gas as the mobile phase.
Thermal Analysis
Thermal analysis techniques, such as TGA and DSC, are used to study the thermal properties of materials and investigate their behavior under different heating or cooling conditions.
- TGA (Thermogravimetric Analysis):Measures the change in mass of a sample as it is heated or cooled, providing information about thermal stability, decomposition, and volatile content.
- DSC (Differential Scanning Calorimetry):Measures the heat flow into or out of a sample as it is heated or cooled, revealing information about phase transitions, melting points, and enthalpy changes.
Data Analysis and Interpretation

Data analysis and interpretation are crucial aspects of chemistry, allowing scientists to extract meaningful information from experimental results. CHEM 1211 emphasizes the development of these skills through various methods.
One method involves graphical representation of data. Creating graphs, such as line graphs, bar charts, and scatter plots, helps visualize trends and patterns in the data. By examining the shape, slope, and intercepts of these graphs, scientists can identify relationships between variables and draw conclusions.
Statistical Analysis
Statistical analysis is another essential tool for data interpretation. Techniques like mean, median, standard deviation, and t-tests provide quantitative measures of central tendency and data variability. These statistics help determine the reliability and significance of experimental results, allowing scientists to make informed conclusions.
Error Analysis
Error analysis is vital in chemistry, as all measurements contain some degree of uncertainty. Understanding the sources and types of errors (systematic and random) enables scientists to assess the accuracy and precision of their data. By applying error propagation techniques, they can calculate the uncertainty in calculated values, ensuring the validity of their conclusions.
Study Resources
Effective preparation for the ACS final exam requires a comprehensive approach, leveraging various study resources to reinforce your understanding of key concepts and problem-solving techniques. This section provides a detailed list of resources to guide your exam preparation.
Textbooks
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy
- General Chemistryby Raymond Chang and Kenneth A. Goldsby
- Chemistry: A Molecular Approachby Nivaldo J. Tro
Online Materials
- ACS Exam Study Guide: https://www.acs.org/content/dam/acsorg/about/governance/committees/testing/acs-exams/study-guides/2023-ACS-Exam-Study-Guide.pdf
- Khan Academy Chemistry: https://www.khanacademy.org/science/chemistry
- Crash Course Chemistry: https://www.youtube.com/playlist?list=PL8dPuuaLjXtOfse2ncvffeelTrqvhrz8H
Practice Problems
- ACS Exam Practice Problems: https://www.acs.org/content/dam/acsorg/about/governance/committees/testing/acs-exams/practice-exams.pdf
- Chemistry LibreTexts Practice Problems: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)/10%3A_Review_of_Essential_Mathematics/10.10%3A_Practice_Problems
- AP Chemistry Practice Problems: https://apcentral.collegeboard.org/courses/ap-chemistry/exam/practice-questions
Other Helpful Resources, Chem 1211 acs final exam
- ACS Exam Information: https://www.acs.org/content/acs/en/careers/college-to-career/tests.html
- Chemistry Tutoring Services: https://www.khanacademy.org/science/chemistry/chem-help
- Study Groups: Connect with classmates or form study groups to discuss concepts and practice problems together.
To effectively utilize these resources, it is recommended to:
- Review the ACS Exam Study Guide to understand the exam format and content areas.
- Read your textbook and supplement your understanding with online materials and videos.
- Practice solving problems from various sources to improve your problem-solving skills.
- Seek help from tutors or study groups when needed to clarify concepts or address specific questions.
- Create a study schedule and stick to it to ensure consistent progress and retention.
Exam Structure and Format
The ACS final exam for CHEM 1211 consists of multiple-choice questions and free-response questions.
The multiple-choice questions cover a wide range of topics from the entire semester, including concepts and theories, problem-solving strategies, experimental techniques, and data analysis and interpretation. The free-response questions typically require students to demonstrate their understanding of a particular concept or theory by applying it to a new situation or solving a problem.
Time Management and Pacing
The ACS final exam is a timed exam, so it is important to manage your time wisely. Here are some tips for pacing yourself during the exam:
- Read the instructions carefully before starting the exam.
- Start with the questions that you are most confident about.
- Don’t spend too much time on any one question. If you are stuck, move on to the next question and come back to it later.
- Use the remaining time to check your answers and make any necessary corrections.
FAQ Resource: Chem 1211 Acs Final Exam
What is the structure of the CHEM 1211 ACS Final Exam?
The exam typically consists of multiple-choice questions, short answer questions, and problem-solving questions.
How can I effectively prepare for the exam?
Attend lectures, review class notes, solve practice problems, and utilize the study resources provided in this guide.
What are some common misconceptions to avoid during the exam?
Be cautious of oversimplifying concepts, neglecting units, and making assumptions without proper justification.