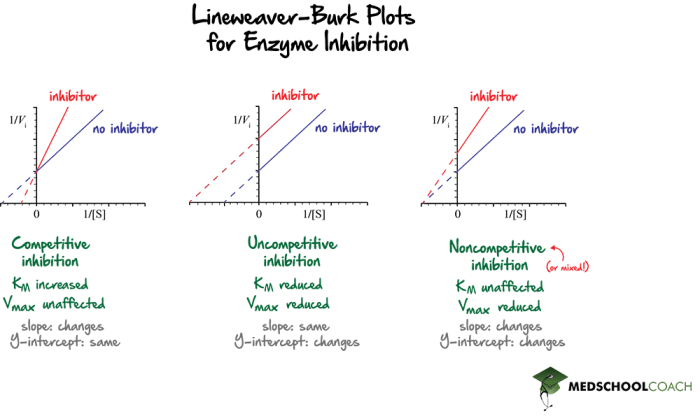
Competitive inhibitor lineweaver burk plot – The competitive inhibitor Lineweaver-Burk plot emerges as a powerful tool in enzyme kinetics, providing a graphical representation of how competitive inhibitors modulate enzyme-substrate interactions. This plot unravels the intricate interplay between enzymes, substrates, and inhibitors, revealing valuable insights into enzyme inhibition mechanisms and their applications in drug design and development.
Competitive inhibitors, by binding reversibly to the active site of an enzyme, introduce a competitive element in enzyme-substrate binding, altering the enzyme’s kinetic behavior. The Lineweaver-Burk plot, with its characteristic intersecting lines, captures this altered behavior, enabling researchers to determine the inhibition constant (Ki) and gain a deeper understanding of enzyme inhibition.
1. Introduction
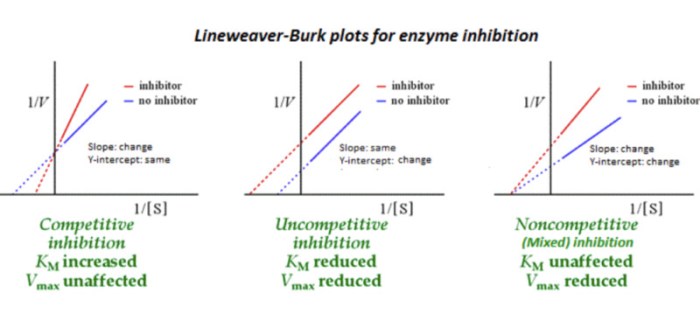
Competitive inhibition occurs when a molecule (inhibitor) binds to the active site of an enzyme and competes with the substrate for binding. This type of inhibition is reversible and can be overcome by increasing the substrate concentration.
The Lineweaver-Burk plot is a graphical representation of enzyme kinetics that can be used to study competitive inhibition. The plot shows the relationship between the reaction rate and the substrate concentration. In the presence of a competitive inhibitor, the Lineweaver-Burk plot will have a higher slope and a lower intercept than in the absence of the inhibitor.
2. Enzyme-Substrate Binding
Competitive inhibitors bind to the active site of an enzyme in a manner that is similar to the substrate. This prevents the substrate from binding to the enzyme, which in turn reduces the reaction rate.
The affinity of a competitive inhibitor for the enzyme is measured by the inhibition constant (Ki). The Ki is the concentration of the inhibitor that reduces the reaction rate by 50%.
3. Lineweaver-Burk Plot Interpretation
The Lineweaver-Burk plot is a useful tool for studying competitive inhibition. The slope of the plot is equal to the Michaelis constant (Km), which is the substrate concentration at which the reaction rate is half of the maximum rate.
The intercept of the plot is equal to the maximum reaction rate (Vmax). In the presence of a competitive inhibitor, the Km will increase and the Vmax will decrease.
4. Determination of Inhibition Constant (Ki)
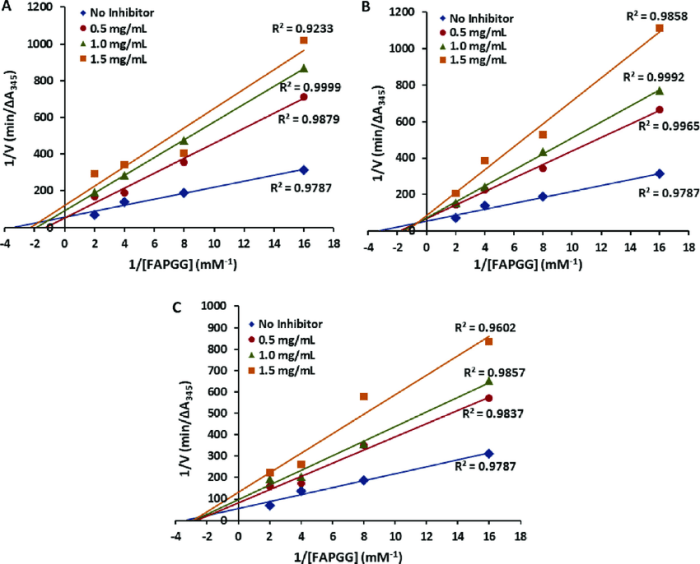
The inhibition constant (Ki) can be determined using a Lineweaver-Burk plot. The Ki is the concentration of the inhibitor that reduces the reaction rate by 50%.
To determine the Ki, a series of Lineweaver-Burk plots are generated at different concentrations of the inhibitor. The Ki is then calculated from the slope and intercept of the plots.
5. Comparison with Non-Competitive Inhibition, Competitive inhibitor lineweaver burk plot
Competitive inhibition is different from non-competitive inhibition. Non-competitive inhibitors bind to the enzyme at a site other than the active site. This type of inhibition is not overcome by increasing the substrate concentration.
In a Lineweaver-Burk plot, non-competitive inhibition will result in a higher Km and a lower Vmax, but the lines will intersect at the same point on the y-axis.
General Inquiries: Competitive Inhibitor Lineweaver Burk Plot
What is the significance of the Lineweaver-Burk plot in competitive inhibition studies?
The Lineweaver-Burk plot provides a graphical representation of enzyme inhibition dynamics, allowing researchers to determine the inhibition constant (Ki) and analyze the effects of competitive inhibitors on enzyme kinetics.
How does the Lineweaver-Burk plot differ in the presence of competitive inhibitors?
In the presence of competitive inhibitors, the Lineweaver-Burk plot exhibits intersecting lines, with an increased slope and a decreased y-intercept compared to the plot without inhibitors.
What is the role of the inhibition constant (Ki) in understanding enzyme inhibition?
The inhibition constant (Ki) represents the concentration of the inhibitor at which the enzyme activity is reduced by half. It provides insights into the strength of the inhibitor-enzyme interaction and the inhibitor’s affinity for the enzyme’s active site.