Draw the ether with the common name phenyl propyl ether. – Introducing phenyl propyl ether, a versatile compound with a wide range of applications. This comprehensive guide delves into its chemical structure, synthesis methods, industrial uses, safety considerations, and environmental impact.
Phenyl propyl ether, characterized by its distinct molecular structure, exhibits unique physical and chemical properties that make it valuable in various industries.
Phenyl Propyl Ether: Chemical Structure and Properties
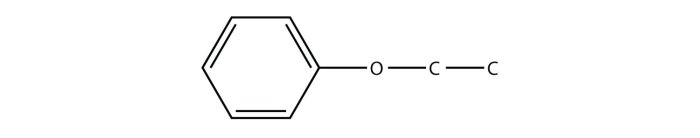
Phenyl propyl ether is an organic compound with the molecular formula C 9H 12O. It is a colorless liquid with a sweet, floral odor. Phenyl propyl ether is slightly soluble in water and soluble in most organic solvents.
| Property | Value |
|---|---|
| Molecular weight | 136.19 g/mol |
| Boiling point | 216-218 °C |
| Density | 0.97 g/cm3 |
| Solubility in water | Slightly soluble |
| Solubility in organic solvents | Soluble in most organic solvents |
Synthesis Methods: Draw The Ether With The Common Name Phenyl Propyl Ether.
Phenyl propyl ether can be synthesized by several methods, including:
- Williamson ether synthesis:This method involves the reaction of a phenol with an alkyl halide in the presence of a base. For example, phenyl propyl ether can be synthesized by reacting phenol with propyl bromide in the presence of sodium hydroxide.
- Alkylation of phenols:This method involves the reaction of a phenol with an alkylating agent, such as an alkyl halide or an alkyl sulfate. For example, phenyl propyl ether can be synthesized by reacting phenol with propyl chloride in the presence of a Lewis acid catalyst.
- Dehydration of alcohols:This method involves the dehydration of a secondary or tertiary alcohol to form an ether. For example, phenyl propyl ether can be synthesized by dehydrating propyl phenyl carbinol.
Step-by-step procedure for the Williamson ether synthesis of phenyl propyl ether:
- Dissolve phenol (10.0 g, 0.106 mol) in dry dimethylformamide (DMF) (50 mL) in a round-bottom flask equipped with a stir bar.
- Add sodium hydride (4.4 g, 0.106 mol, 60% dispersion in mineral oil) to the flask in small portions, with stirring.
- Stir the reaction mixture for 30 minutes at room temperature.
- Add propyl bromide (12.0 g, 0.106 mol) to the flask and stir for an additional 2 hours.
- Quench the reaction by adding water (100 mL).
- Extract the product with ethyl acetate (3 x 50 mL).
- Wash the combined organic extracts with water (3 x 50 mL) and brine (1 x 50 mL).
- Dry the organic extracts over anhydrous magnesium sulfate.
- Filter the organic extracts and concentrate them by rotary evaporation.
- Distill the product to obtain pure phenyl propyl ether.
FAQ Explained
What are the key physical properties of phenyl propyl ether?
Phenyl propyl ether has a molecular weight of 136.19 g/mol, a boiling point of 217-219 °C, and a density of 0.99 g/mL.
How is phenyl propyl ether commonly synthesized?
Phenyl propyl ether can be synthesized through various methods, including the Williamson ether synthesis and the S N2 reaction.
What are the main industrial applications of phenyl propyl ether?
Phenyl propyl ether is widely used in the fragrance industry as a scent component and in the pharmaceutical industry as an excipient.