Unit chemical quantities mole conversions with volume ws 4 – Embark on a comprehensive exploration of unit chemical quantities, delving into the intricacies of mole conversions with volume. This in-depth analysis unveils the fundamental concepts, practical applications, and advanced nuances of this essential chemical technique.
Unit chemical quantities provide a standardized framework for expressing the amounts of substances in chemical reactions. Mole conversions, a cornerstone of quantitative chemistry, enable the seamless conversion between mass, volume, and moles, facilitating precise calculations and accurate experimentation.
1. Unit Chemical Quantities
Mole Conversions with Volume
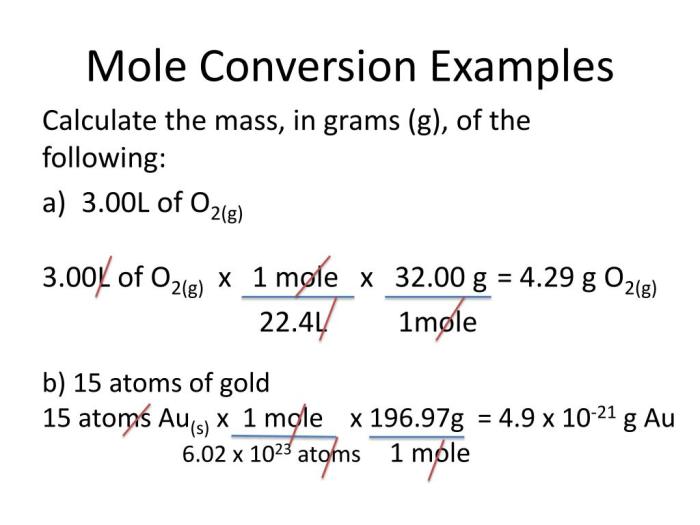
In chemistry, mole conversions play a crucial role in relating the amount of a substance to its volume. Mole conversions involve converting between the number of moles of a substance and its volume, typically expressed in liters (L).
Understanding these conversions is essential for accurate calculations in various chemical applications.
Calculations and Formula Applications, Unit chemical quantities mole conversions with volume ws 4
The formula for mole conversions involving volume is:
Moles (mol) = Volume (L) × Concentration (mol/L)
where:
- Moles (mol) is the amount of substance in moles.
- Volume (L) is the volume of the solution in liters.
- Concentration (mol/L) is the concentration of the solution in moles per liter.
Using this formula, we can calculate the number of moles of a substance given its volume and concentration, or vice versa.
Applications in Chemistry
Mole conversions with volume are widely used in chemistry, including:
- Preparing solutions of specific concentrations.
- Determining the amount of reactant required for a reaction.
- Calculating the volume of a solution needed to react with a certain amount of substance.
Popular Questions: Unit Chemical Quantities Mole Conversions With Volume Ws 4
What is the significance of using the correct units in mole conversions?
Using the correct units ensures accurate and meaningful calculations. Mismatched units can lead to erroneous results and hinder the interpretation of experimental data.
How are mole conversions applied in real-world chemistry settings?
Mole conversions are essential in preparing solutions of specific concentrations, determining the limiting reactant in reactions, and calculating the yield of products.
What are the limitations of mole conversions with volume?
Mole conversions with volume assume ideal behavior of solutions and do not account for non-idealities such as deviations from linearity or temperature effects.